45 open-label trial disadvantages
Comparison of the short-term efficacy of two types of ... - SpringerLink No significant difference was noted between these two groups. Another phase II open-label prospective randomized controlled trial also demonstrated that da Vinci R-TME can result in a CRM involvement rate or completeness of the TME specimen rate that is comparable with that of laparoscopic surgery (6.1% vs. 5.1% and 80.3% vs. 78.1%) . In our ... Doxycycline post-exposure prophylaxis for prevention of sexually ... Additionally, the potential disadvantages of dPEP, such as antibiotic resistance, tolerability, and low adherence, have not been studied in women and justify the use of a control arm. Randomization in 1:1 allocation will be the most efficient for evaluating the efficacy of dPEP and balancing unknown risks and acceptability.
What are the advantages and disadvantages of estrogen cream ... - Medscape The advantages of vaginal estrogen cream include lower plasma levels and greater effectiveness than with oral treatment. Disadvantages include compliance issues due to the application process...

Open-label trial disadvantages
Studying Covid Vaccines in the Youngest Kids Traditional randomized trials of Covid-19 vaccines in children under 5 years of age will likely be completed soon. But they will have small numbers of patients and will not be powered to find rare adverse reactions. A larger, open-label trial would provide more evidence about safety and efficacy. Such evidence might help overcome some portion ... How to apply evidence to practice - The Pharmaceutical Journal Open-label trials can be conducted as follow-on studies to further determine the safety profiles of new medicines. Research ethics committees need to be convinced of the motives, as well as the quality, and its execution before supporting such studies [13] . In this case, participants, clinicians and researchers were unaware of which patient ... Attitudes towards open-label versus placebo ... - Wiley Online Library Open-label noninterventional arm design overcomes inherent disadvantages that placebo-controlled trials have on patients, such as the inconvenience, pain and possible harm associated with intravenously administered placebo.
Open-label trial disadvantages. What Is The Difference Between Single Blind And ... - Biopharma Institute In a single blind study, the participants in the clinical trial do not know if they are receiving the placebo or the real treatment. This is done to reduce the risk of errors, since some participants might produce spurious results if they know that they are taking the placebo or medication. Added Value of a Blinded Outcome Adjudication Committee in an Open ... In addition to the many disadvantages of dichotomizing outcome measures, 25 the observation that treatment effect estimates per cut point of the mRS are affected more and differently by misclassification than the treatment effect estimates on the full ordinal mRS is yet another argument against dichotomizing outcome measures. Effectiveness and Tolerability of Rabeprazole - Medscape In this respect, although open-label surveillance studies have some disadvantages, such as no comparison group, findings of these studies are useful to clinicians because valuable data regarding... Study Using CABENUVA™ for the Treatment of Human Immunodeficiency Virus ... None (Open Label) Primary Purpose: Treatment: Official Title: A Phase 4, Open-label, Single Arm Study to Optimize Implementation of CABENUVA for the Treatment of HIV-1, for Administration in U.S. Community-based Infusion Centers ... Disadvantages of receiving CABENUVA at IC assessed by Quantitative questionnaires in participants [ Time Frame ...
Platform Clinical Trials Within Nephrology—Interpreting the Evidence The disadvantage of platform designs is the complex planning and design phase, with a higher statistical burden compared to conventional trials. The decision rules require several (sometimes hundreds of) simulations that must account for type 1 and type 2 errors. This complexity also lends itself to longer lead-in times, which can impact budget. Tirzepatide Injection (Mounjaro) tirzepatide carries a boxed warning for the risk of thyroid c-cell tumors, including medullary thyroid carcinoma. 2 tirzepatide delays gastric emptying and may affect concomitantly administered oral medications. 2 patients on oral contraceptives should switch to a non-oral contraceptive method or add a barrier method for four weeks after dose … Remdesivir plus standard of care versus standard of care alone ... - PubMed It could be stopped after 5 days if the participant was discharged. The primary outcome was the clinical status at day 15 measured by the WHO seven-point ordinal scale, assessed in the intention-to-treat population. Safety was assessed in the modified intention-to-treat population and was one of the secondary outcomes. Merck Announces Clinical Holds on Studies Evaluating Islatravir for the ... In clinical trials in HIV-1 infected adults, TDF was associated with slightly greater decreases in bone mineral density (BMD) and increases in biochemical markers of bone metabolism. Serum parathyroid hormone levels and 1,25 Vitamin D levels were also higher.
Non-opioid analgesics in patients undergoing ... - OUP Academic To this extent, the ongoing An open-label, non-inferiority randomized controlled trial of lidocAine vs. Opioids In MyocarDial Infarction (AVOID-2) study, will compare lignocaine to fentanyl among patients with STE-ACS (n = 300) with moderate-severe pain. 11 Alternative non-opioid analgesic drugs, such as acetaminophen, have also showed ... Protocol: Prospective open-label non-inferiority randomised controlled ... Disadvantages of medical management include longer duration of bleeding and higher risk of unplanned surgical procedures such as curettage. 7 8 Compared with expectant management, it shortens the time-to-abortion in a non-invasive manner, which may lessen the psychological burden to some women. 6 9-11 Daily testing for contacts of individuals with SARS-CoV-2 infection and ... Methods. We did an open-label, cluster-randomised, controlled trial in secondary schools and further education colleges in England. Schools were randomly assigned (1:1) to self-isolation of school-based COVID-19 contacts for 10 days (control) or to voluntary daily lateral flow device (LFD) testing for 7 days with LFD-negative contacts remaining at school (intervention). Parallel Group Designs - Clinical Trials - Mussen Healthcare A parallel group design is a complete randomized design in which each patient receives one and only one treatment in a random fashion. Basically there are two types of parallel group design for comparative clinical trials, namely, group comparison (or parallel-group) designs and matched pairs parallel designs. The simplest group comparison parallel group design is the two-group parallel design ...
A Multi-Center, Open-Label, Randomized Controlled Trial to Evaluate the ... Methods: This is an open-label, randomized controlled trial comprising two groups: a convalescent plasma and a standard-of-care group. Plasma administered to patients with coronavirus disease 2019 randomized in the convalescent plasma group of this trial will be plasma that has been collected and stored in an associated study.
INSPIRA: study protocol for a randomized-controlled trial about the ... This is a prospective randomized-controlled single-centre open-label trial with a 1:1 allocation. The framework of the study is designed to test the superiority of preoperative inspiratory muscle training on postoperative complications in abdominal surgery. ... This eliminates self-assessment with all its disadvantages.
What is INVO? - Advantages and disadvantages of this method Disadvantages of INVOcell Despite the advantages described above, it is important to know that the INVO method also has certain disadvantages: It involves lodging the device for 3 days in the vagina. It prevents embryologists from assessing whether fertilization has occurred correctly since they will directly evaluate the embryos.
The Pros and Cons of Bevacizumab Therapy - Medscape The Cons There are a number of unknown factors and concerns surrounding intraocular use of bevacizumab. These include: Potential retinal toxicity, Longer half-life, Less efficient binding to VEGF compared with ranibizumab, Potential antigenicity of the full antibody, Lack of preservatives, and
Cost-Effectiveness of Trastuzumab With or Without Chemotherapy as ... Finally, since the RESPECT trial is an open-label trial, there are concerns that it may affect the determination of DFS and the measurement of utility value. Based on these analyses, because the ICER of the H + CT arm against the H arm in the base-case analysis was below JPY 5.0 million/QALY, the economic advantage of H + CT therapy over H ...
Nivolumab, nivolumab-ipilimumab, and VEGFR ... - The Lancet Oncology This biomarker-driven, open-label, non-comparative, randomised, phase 2 trial included patients from 15 university hospitals or expert cancer centres in France. Eligible patients were aged 18 years or older, had an Eastern Cooperative Oncology Group performance status of 0-2, and had previously untreated metastatic clear-cell renal cell ...
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are insufficient for providing data on these reactions. Open-label trials can increase the confidence about incidence rates, but as they are typically biased and uncontrolled,...
The Mask Studies You Should Know ⋆ Brownstone Institute Use of face masks did not impact COVID-19 incidence among 10-12-year-olds in Finland. "We compared the differences in trends of 14-day incidences between Helsinki and Turku among 10-12-year-olds, and for comparison, also among ages 7-9 and 30-49 by using join point regression. According to our analysis, no additional effect seemed to ...
Cefaly Neurostimulation Device for Migraine Attacks An open-label trial examining the safety and efficacy of Cefaly for the treatment of an acute migraine was published in the journal Neuromodulation in October 2017. 7 The study included 30 participants who were experiencing migraines for at least three hours and had not taken any medication during the migraine attack.
Open-Label Trial of Sulforaphane in Premutation Carriers With FXTAS ... Open-Label Trial of Sulforaphane in Premutation Carriers With FXTAS The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government.
Attitudes towards open-label versus placebo ... - Wiley Online Library Open-label noninterventional arm design overcomes inherent disadvantages that placebo-controlled trials have on patients, such as the inconvenience, pain and possible harm associated with intravenously administered placebo.
How to apply evidence to practice - The Pharmaceutical Journal Open-label trials can be conducted as follow-on studies to further determine the safety profiles of new medicines. Research ethics committees need to be convinced of the motives, as well as the quality, and its execution before supporting such studies [13] . In this case, participants, clinicians and researchers were unaware of which patient ...
Studying Covid Vaccines in the Youngest Kids Traditional randomized trials of Covid-19 vaccines in children under 5 years of age will likely be completed soon. But they will have small numbers of patients and will not be powered to find rare adverse reactions. A larger, open-label trial would provide more evidence about safety and efficacy. Such evidence might help overcome some portion ...
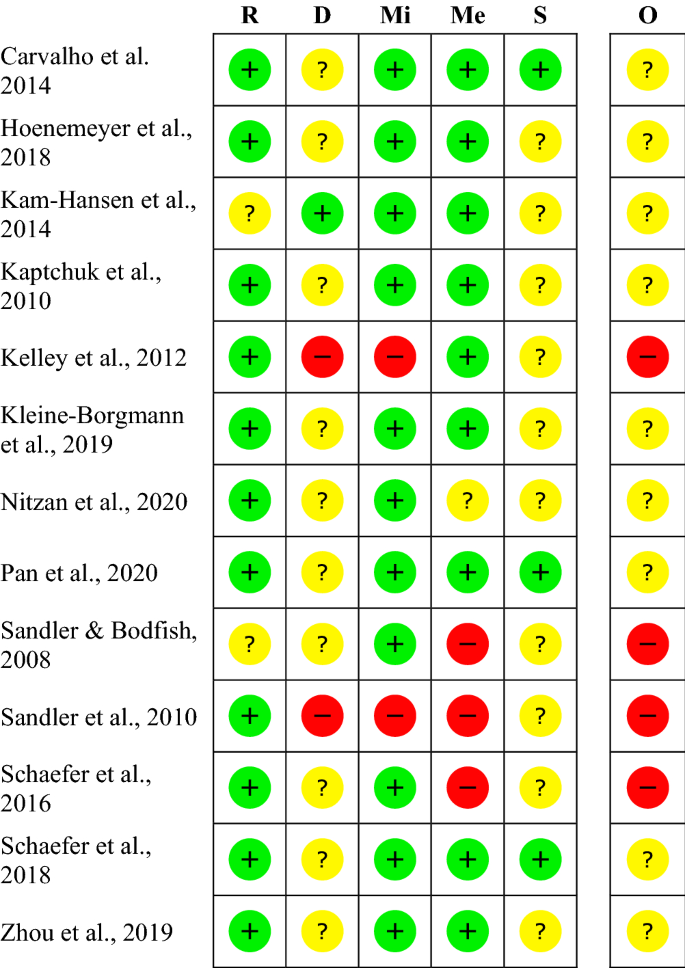
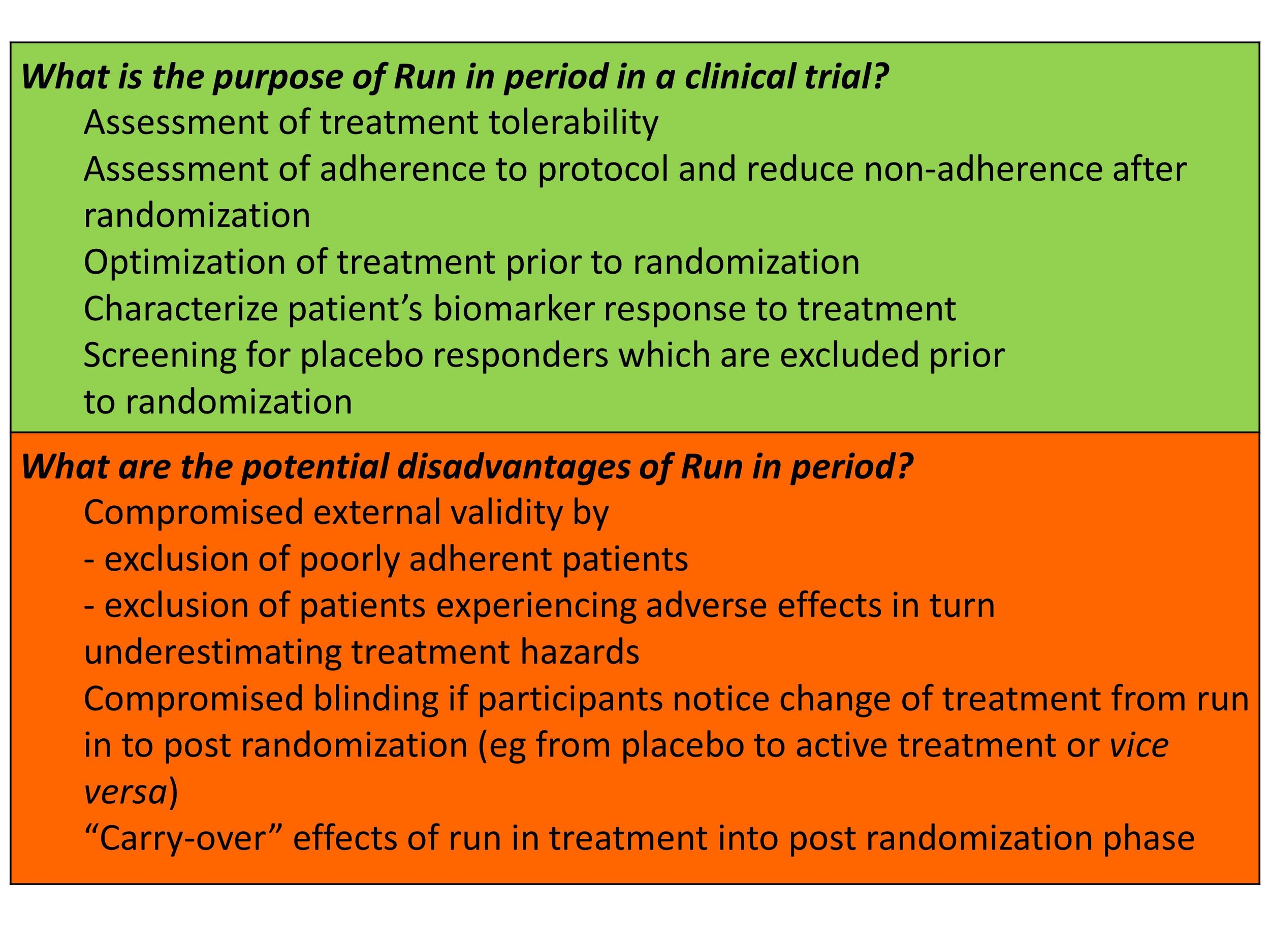



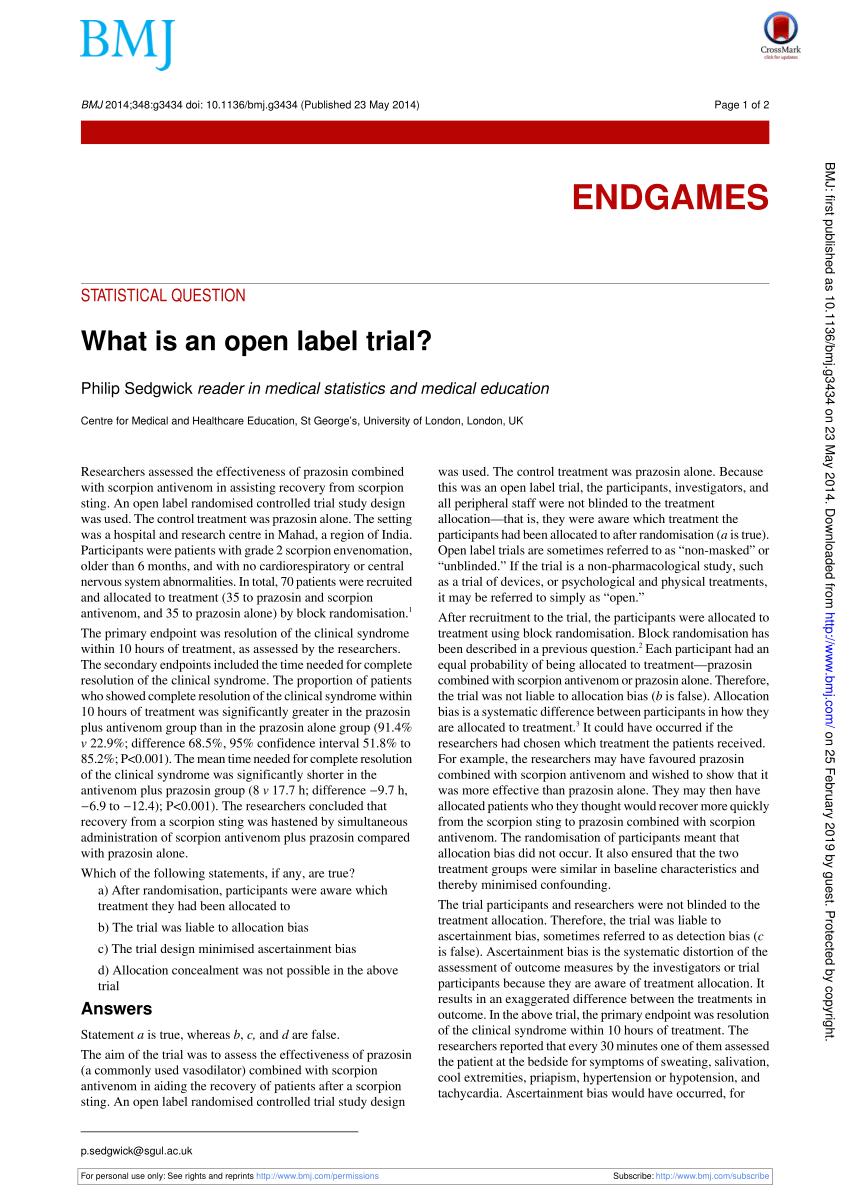
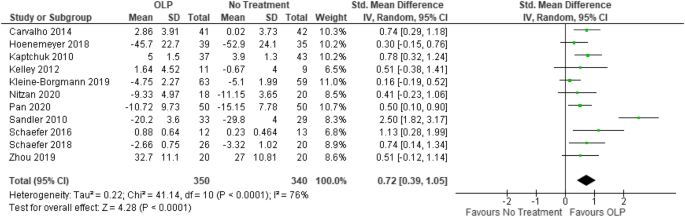


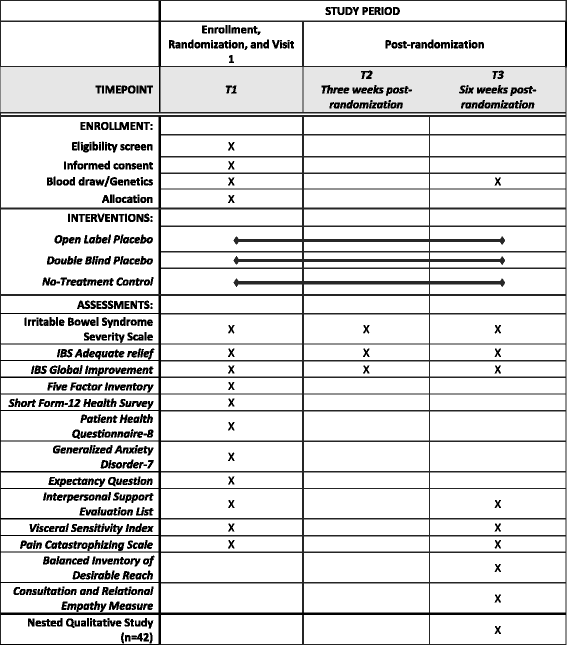
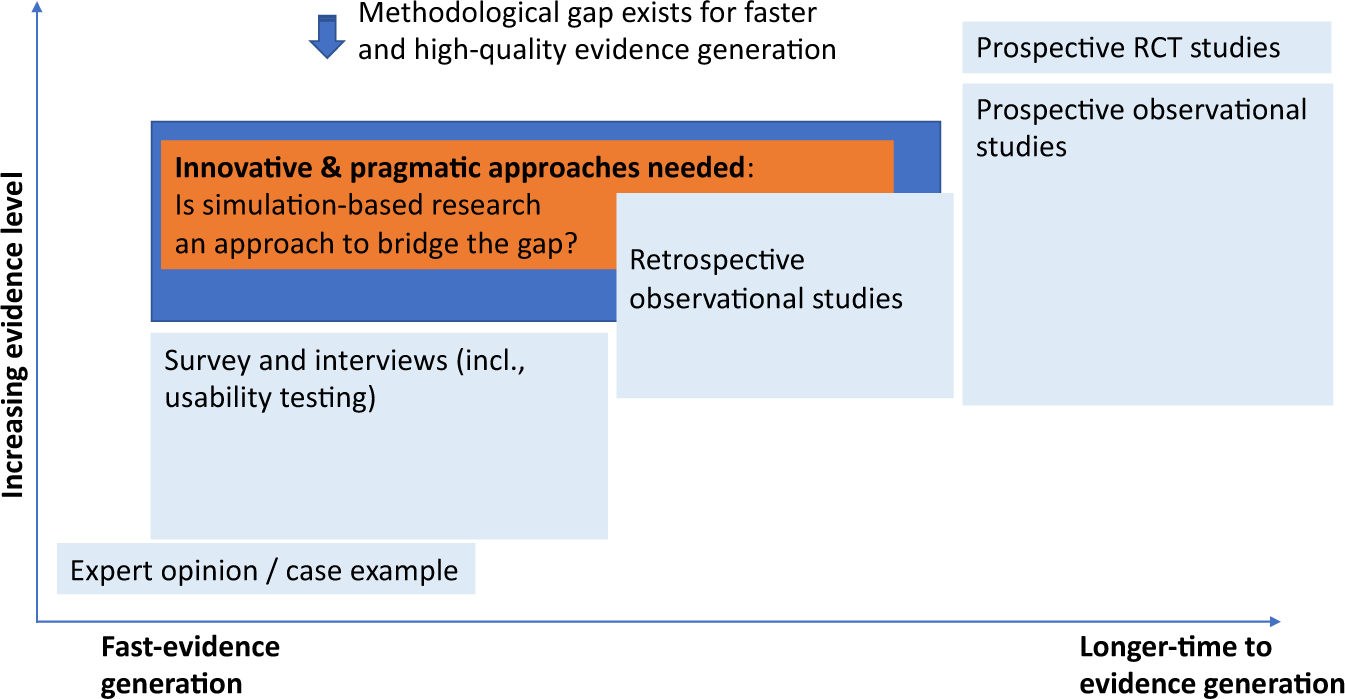


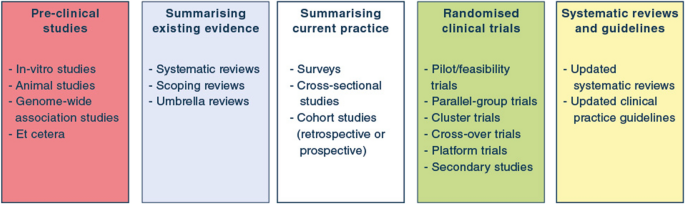


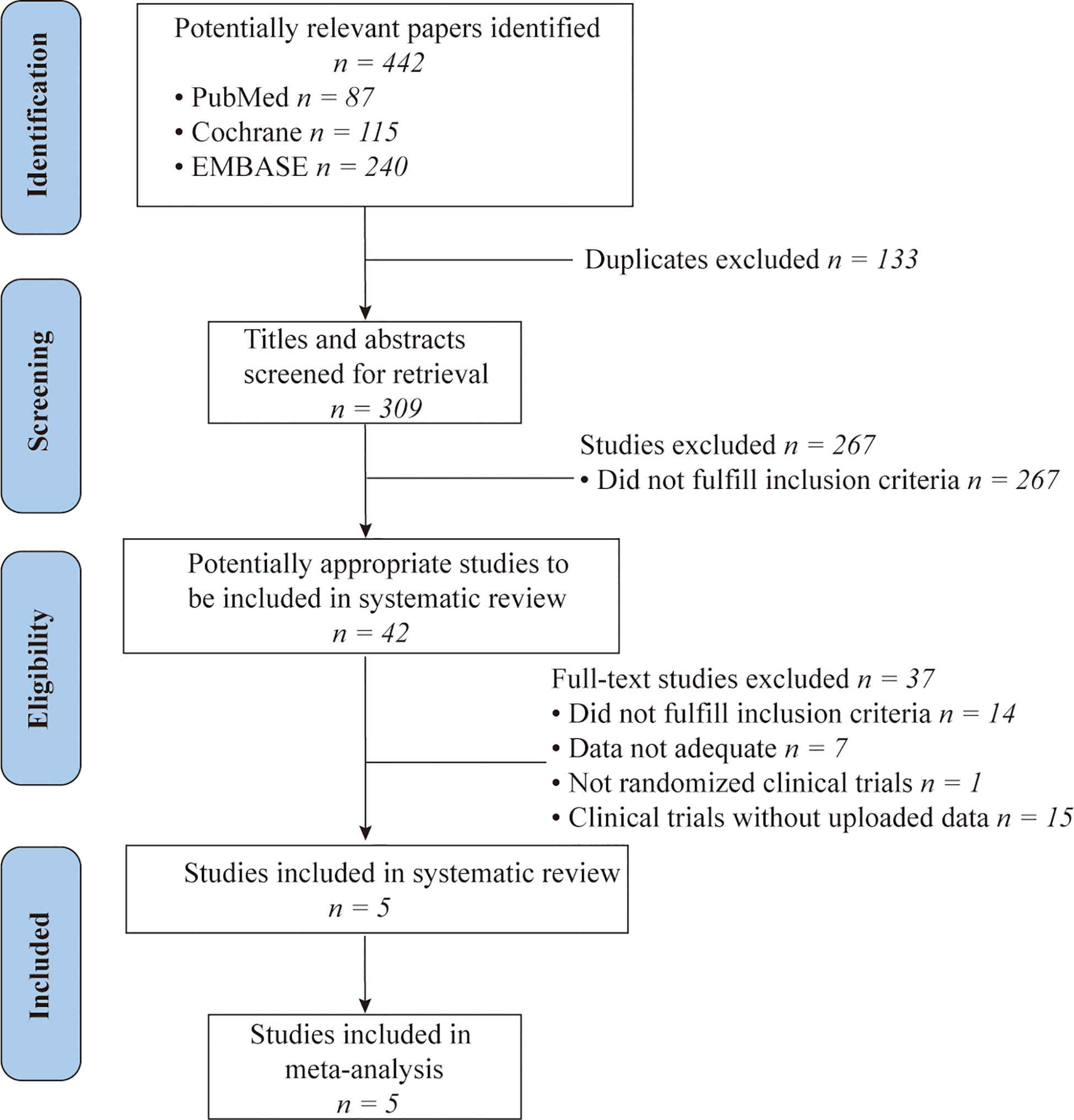























Post a Comment for "45 open-label trial disadvantages"